
Cystoflow System

Device for continuous and manual bladder lavage in a closed circuit for the postoperative period of urological surgery and/or other hematuria situations.
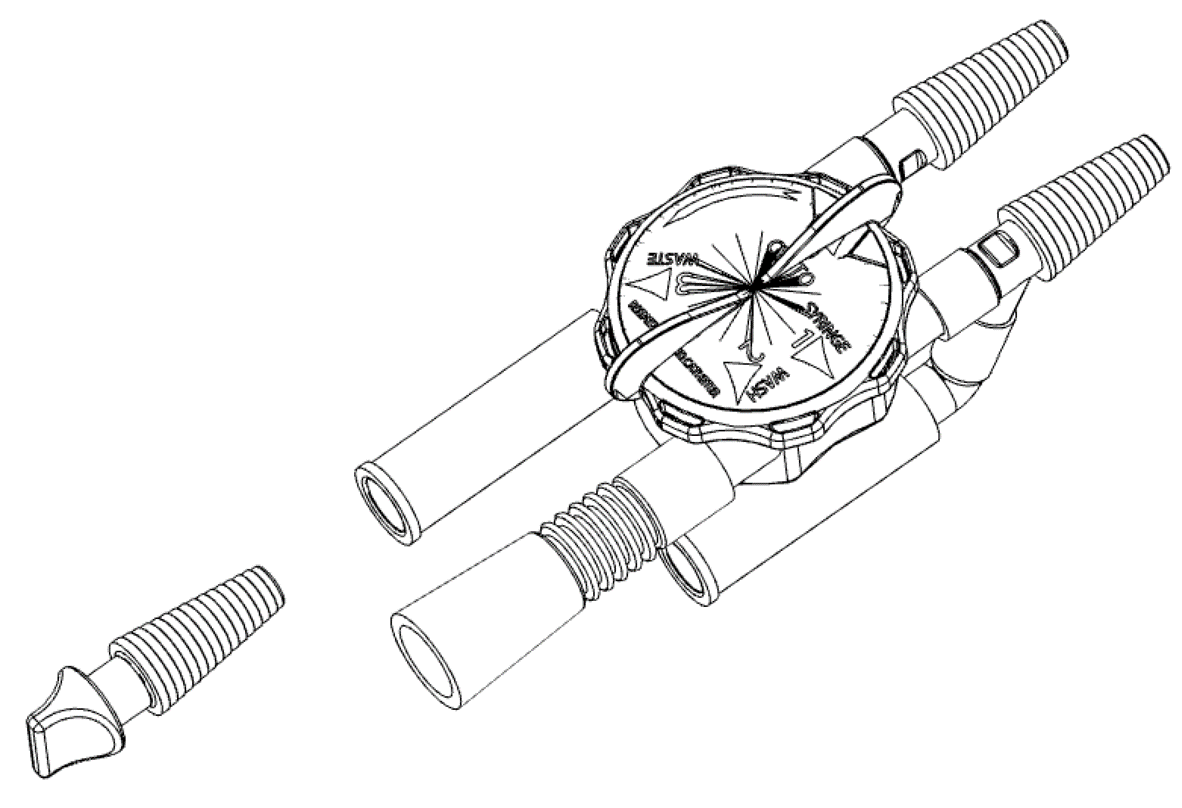
Product Composition
- A rotary selector in white ABS, equipped with a 4-color adhesive label to identify the various positions of the device and a spring-loaded locking pin;
- Main body in white ABS, with 4 ways that includes a polycarbonate fluid diverter that allows 4 operating modes;
- Conical connector in transparent PVC FREE of DEHP, straight and "Y" configuration, for connection to the urinary catheter;
- Funnel-type fittings in DEHP-free PVC, in three different colours: green, for connection to the continuous irrigation/washing system; blue, for syringe connection for manual flushing; yellow, for connection to the bladder drainage bag;
- Spigot in blue polyethylene to occlude the access route for manual washing (syringe);
Indication of Use
Manual and irrigation system bladder lavage in patients with macrohematuria.
Cystoflow is an innovative medical device designed to optimize the management of manual lavage procedures in the presence of macrohematuria. It is characterized by being a closed-loop system at all stages of its use, clean, safe and easy and intuitive to manage. Stay in the patient – 15 days.
Packaging
Primary packaging: one device packed in microperforated PE, outer packaging in medical grade paper/PP-PE film.
Secondary packaging: box with 15 pieces; Tertiary packaging box with 60 pieces (4x15 units).
Production process
The device is manufactured in accordance with the Quality System of HMC Premedical S.p.A. and complies with the requirements of EN ISO 13485.
Product Control
All production phases are carried out in accordance with internal procedures and sampling plans defined by the ISO 2859-1 standard.
Classification
Class IIa sterile.
Sterilization
Sterilized with ethylene oxide in accordance with current regulations. Shelf life: 5 years from the date of sterilization. Single use. Not resterilizable.